We use various types of materials in our daily life. Those materials are made up of various materials like wood, glass, plastic, thread, soil, metals, rubber, etc. Out of those, wood, rock, minerals, water are natural. Human performed research on various natural materials in laboratory. With the help of it, various materials are manufactured in factories. Such materials are called as manmade materials. eg. Glass, plastic, artificial threads, thermocol, etc.
 Plastic
Plastic
A manmade material showing the property of plasticity and made up of organic polymers is plastic. Structure of all the plastics is not same. Some are linear while some are circular. Depending upon the effect of heat, plastic can be classified into two types. The plastic that can be molded as per our wish is called as thermoplastic. eg. Polythene, PVC are used for manufacturing the toys, combs, plates, bowls etc. Another plastic is such that once a specific shape is given with the help of mold, its shape cannot be changed on heating. It is called as thermosetting plastic. eg. Electric switches, coverings over the handles of cookers, etc.
Properties of Plastic: Plastic does not corrode. It does not decompose. It is not easily affected by humidity, heat, rain, etc. Items of any colour can be made from it. It can be molded into any shape due to the property of plasticity. It is bad conductor of heat and electricity. Being light in weight, it is easy to carry.
Plastic and environment
- How many plastic carry bags are brought in your home in a day? What happens to those later on?
- How are the used up and thrown away carry bags, water bottles, milk bags recycled?
Some materials are naturally degraded, they are called as degradable materials while some materials do not; called as non-degradable material. From the given on next page chart, we can understand that plastic is nondegradable and hence it is an environment pollutant. Which measures can we arrange to avoid this?
 Thermocol : A new, easily breakable item brought at your home is usually packed in a box. So as to prevent that item from breaking while handling the box, it is always packed in one more wrapping. Usually, that wrapping is of thermocol.
Thermocol : A new, easily breakable item brought at your home is usually packed in a box. So as to prevent that item from breaking while handling the box, it is always packed in one more wrapping. Usually, that wrapping is of thermocol.
Now a day, the plates used in mass feasts are also made up of thermocol. Thermocol is a form of a complex material called polystyrene. It transforms in to liquid state on heating at more than 100 ºC temperature and returns to solid state on cooling. Due to this, we can give any desired shape to it. Being a good shock-absorber, it is used in packing of delicate items. Make list about use of thermocol in your daily life
Adverse effects of excessive use of thermocol on environment and human:
- Being carcinogenic ingredients in styrene, the person in contact with thermocol for long duration may have the possibility of blood cancer like leukemia and lymphoma.
- Non-biodegradable : It takes long duration for natural degradation of thermocol; hence many people opt for destroying it by burning. However, it is still more hazardous method as it releases poisonous gases in atmosphere.
- In mass gatherings, plates and cups used to offer the food, water, tea are made up of thermocol. It affects the health. If the food kept in thermocol is reheated, styrene may dissolve in that food. Due to this, there is possibility of health problem.
- Effect on persons working in thermocol factory : Persons staying in contact with thermocol for long term may develop the problems of eyes, respiratory system, skin, digestive system, etc. Pregnant women may face the miscarriage. Liquid styrene may cause skin-burns.
Glass : 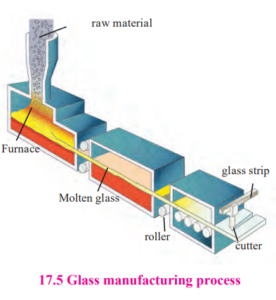 We use the glass material on large scale in our daily life. Glass was discovered by chance. Some Phoenician traders were cooking in desert. The cooking vessels were supported on lime-stones. When the cooking vessel was kept off the lime-stone, they observed that a transparent material has been formed. They thought that this transparent material may had been formed due to heating together of sand and limestone. This led to the development of technique of glass production. Glass is the non-crystalline, hard but brittle solid material formed from mixture of silica and silicate. Silica i.e. SiO2 to which we refer to as sand. Depending upon the proportion of silica and other components in the glass; there are different types of glass as soda-lime glass, boro-silicate glass, silica glass, alkalisilicate glass, etc.
We use the glass material on large scale in our daily life. Glass was discovered by chance. Some Phoenician traders were cooking in desert. The cooking vessels were supported on lime-stones. When the cooking vessel was kept off the lime-stone, they observed that a transparent material has been formed. They thought that this transparent material may had been formed due to heating together of sand and limestone. This led to the development of technique of glass production. Glass is the non-crystalline, hard but brittle solid material formed from mixture of silica and silicate. Silica i.e. SiO2 to which we refer to as sand. Depending upon the proportion of silica and other components in the glass; there are different types of glass as soda-lime glass, boro-silicate glass, silica glass, alkalisilicate glass, etc.
Production of Glass : For glass production, mixture of sand, soda, lime, and small quantity of magnesium oxide is heated in furnace. Sand i.e. silicon dioxide melts at 1700 0 C. So as to melt the mixture at low temperature, pieces of discarded glass are added to it. Due to this, mixture melts at 850 ºC. Once all the ingredients of mixture are liquified, it is heated up to 1500 ºC and immediately cooled. Due to sudden cooling, mixture becomes homogenous, amorphous and transparent instead of crystalline. This is called Soda-lime glass.
Properties of Glass :
1. On heating, glass becomes soft and can be moulded into any shape.
2. Density of glass depends upon its ingredients.
3. Glass is slow conductor of heat. On quick heating of cool glass or on quick cooling of hot glass, it cracks / breaks.
4. Being bad conductor of electricity, glass is used as insulator in electric appliances.
5. Being transparent, most of light passes through the glass. However, if there are oxides of either chromium, vanadium or iron in the glass, large amount of light is absorbed in glass.
Types of Glass and Uses:
- Silica glass : This is produced by using the silica. Items made up of silica glass show minimum expansion on heating. It is not affected by acid and alkali. Due to this, silica glass is used to produce laboratory glass-wares.
- Borosilicate glass : Borosilicate glass is produced by melting the mixture of sand, soda, boric acid and aluminium oxide. This glass does not show any effect on medicines. Hence, the bottles made up of borosilicate glass are used in pharmaceutical industry to store the medicines.
- Alkali silicate glass : Alkali silicate glass is produced by heating the mixture of sand and soda. As this glass is soluble in water, it called as ‘water glass’.
- Lead glass : Lead glass is produced by melting the mixture of sand, soda, limestone and lead oxide. Being very clear / transparent, it is used in manufacturing of light bulbs, tubes, etc.
- Optical glass : Optical glass is produced from the mixture of sand, soda, limestone, barium oxide and boron. This type of pure glass is useful in production of spectacles, lenses, microscopic lenses, etc.
- Coloured glass : Soda lime glass is colorless. So as to impart a desired colour, oxide of specific metal is mixed during manufacturing process. eg. Ferrous oxide is mixed to get bluish green glass and copper oxide to get red glass.
- Processed glass : So as to improve the quality and utility, some processing is performed on glass and various types like reinforced glass, plain glass, fiber glass, fen glass, translucent glass, etc. are produced.
